Section: New Results
Tissue growth, regeneration and cell movements
Chemotaxis, self-organisation of cell communities
Participants : Nikolaos Bournaveas [Univ. Edinburgh] , Axel Buguin [UPMC, Institut Curie] , Vincent Calvez [ENS Lyon] , François James [univ. Orléans] , Alexander Lorz, Grégoire Nadin [UPMC] , Benoît Perthame, Jonathan Saragosti [Institut Curie] , Pascal Silberzan [Institut Curie] , Min Tang [SJTU] , Nicolas Vauchelet.
We have continued our analysis and simulation of models for large bacterial communities and more generally cells self-organisation as inititated several years ago [12] , [31] . This is a rich domain because on the one hand several Partial Differential Equations arise, parabolic models, kinetic equations, hyperbolic systems and on the other hand complex patterns occur that are a sign of the complex underlying dynamics.
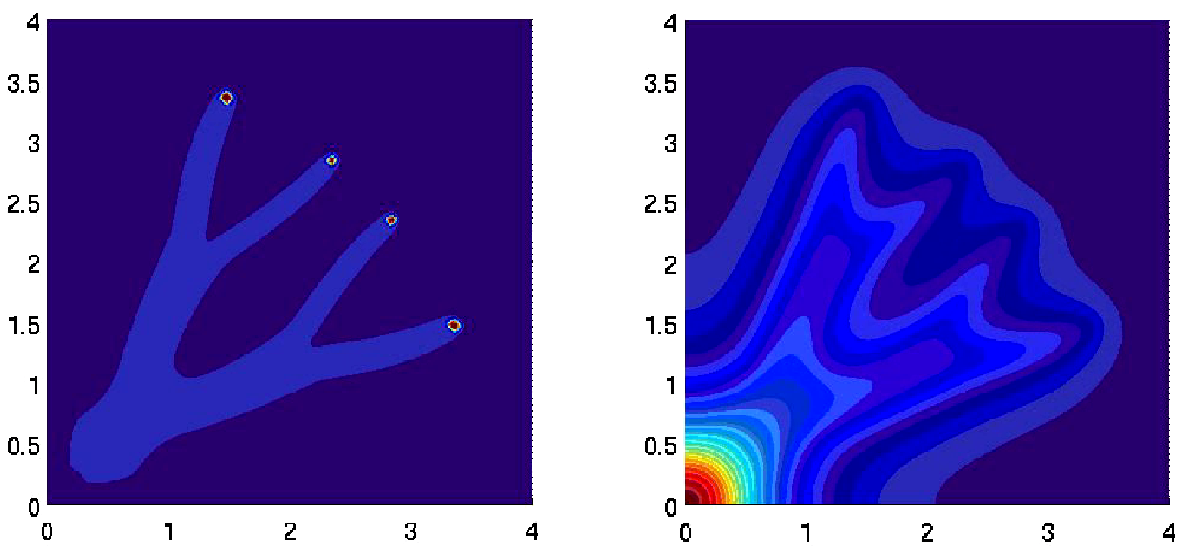
In their article [65] , Y. Dolak and C. Schmeiser have proposed a mathematical model describing the individual behavior of bacteria responding to a chemical substance. Numerical simulations of this kinetic model have been investigated in [93] . In a macroscopic level of description, we perform a hydrodynamical limit which leads to an aggregation model, for which regular solutions blows up in final time [82] . The rigorous study of the behavior of the aggregates relies on a careful analysis of some non-linear scalar conservation laws in the framework of measures [24] and has been investigated in [55] .
|
With the group of P. Silberzan in Curie Institute, in 2010 we have given an explanation of traveling bands first observed by Adler in the 80's for E. coli in microchannels. In a continuation of this work, based on analysis of individual trajectories, we have shown directional persistence which improves the efficiency of collective migration. Kinetic models with tumbling kernels that keep memory of the incoming velocity are able to reproduce accurately the wave parameters [35] .
Computing effectively traveling wave can be difficult in unstable cases. An algorithm is proposed in [29] for the NonLocalFisher equation which catches the traveling wave and not the more stable pulsating wave.
A. Lorz has studied a system consisting of the elliptic-parabolic Keller–Segel equations coupled to Stokes equations by transport and gravitational forcing. We show global-in-time existence of solutions for small initial mass in 2D. In 3D we establish global existence assuming that the initial -norm is small. Moreover, we give numerical evidence that for this extension of the Keller–Segel system in 2D, solutions exist with mass above , which is the critical mass for the system without fluid. The model is written as
Here denotes the concentration of a chemical, a cell density and a fluid velocity field described by Stokes equations. The fluid couples to and through transport and gravitational forcing modelled by . The pressure can be seen as the Lagrange multiplier enforcing the incompressibility constraint. The chemical diffuses, it is produced by the cells and it degrades. The cell density diffuses and it moves in the direction of the chemical gradient. The constant measures self-degradation of the chemical and the constants , determine the evolution undergone by .
Single cell-based models of tumour growth, tissue regeneration, embryonic development
Participants : Annabelle Ballesta, Gregory Batt [CONTRAINTES project-team] , François Bertaux, Chadha Chettaoui, Ibrahim Cheddadi, Dirk Drasdo, Adrian Friebel, Rolf Gebhardt [Univ. of Leipzig, Germany] , Adriano Henney [Director Virtual Liver Network and VLN consortium] , Jan G. Hengstler [Leibniz Research Center, Dortmund, Germany and CANCERSYS consortium] , Stefan Höhme, Elmar Heinzle [University of Saarbrücken and NOTOX consortium] , Isabelle Hue [INRA] , Nick Jagiella, Ursula Klingmüller [German Cancer Center, Heidelberg and LungSys Consortium] , Axel Krinner, Emanuele Leoncini, Johannes Neitsch, Benoît Perthame, Ignacio Ramis-Conde, Luc Soler [ IRCAD, Coordinator EU-project PASSPORT and PASSPORT consortium] , Irène Vignon-Clémentel [REO project-team] , Juhui Wang [INRA] , William Weens.
Structure formation in tissues as well as malfunctions on the multi-cellular level are inherently of multi-scale nature. Modifications on the molecular level by intrinsic or extrinsic factors affect the architecture and function on the multi-cellular tissue level. Much of the current research so far focuses on the analysis of intracellular pathways, genetic and metabolic regulation on the intracellular scale and on continuum equations for local densities of cells to capture multi-cellular objects on large spatial scales but only recently have increasing efforts been made at the interface between these two: individual cell based models (IBMs) which permit to include the molecular information on the one hand and to extrapolate to the multi-cellular tissue level on the other hand and hybrid models that combine continuum with individual-based models for different components.
|
|
In order to fill the existing gap we have studied intracellular regulation networks [87] , [72] , multi-scale IBMs where intracellular regulation and differentiation was explicitly represented within each individual cell [90] , [85] , [91] , lattice-free IBMs [69] and continuum models that can capture their large scale behaviour [63] , and cellular automaton (CA) models where each lattice site can be occupied either by at most one cell [61] or by many cells [89] , [71] and their corresponding continuum equation [68] . Moreover, for a simple, but for rigorous coarse graining not accessible, growth situation we were able to obtain quantitatively matching results with continuum and individual-cell-based models without any fit parameter.
Besides the methodical aspects we focus on a number of applications:
-
Unstructured cell populations growing in a monolayer with free border [69] , [74] , or constraint by the presence of a granular or cellular embedding medium [19] .
-
Multicellular spheroids in liquid suspension [69] , [70] , and embedding granular or cellular matter [19] . For non-small-lung-cancer cell lines growing as multi-cellular spheroids, we could, starting with a complete parameterisation of the model by labelling experiments, simultaneously explain the proliferation, apoptosis, extracellular matrix and growth pattern of multi-cellular spheroids under different nutrient conditions within one consistent mathematical model.
-
Vascular tumour growth.
-
Regulatory and evolutionary aspects in tumour growth [81] , [78] , recently with resolution of intracellular signal transduction pathway variants found in normal vs. malignant cells [33] .
-
Cell differentiation and lineage commitment of mesenchymal stem cells [85] , [73] . In our earlier work we have established a model of cell aging for in-vitro cultured stem cell populations. Stem cell concepts developed earlier [85] , [73] have been extended to include cell aging [83] . By this extension it is possible to explain the clonal heterogeneity that was not captured by the previous model. The cell age was coupled with the generation number. It is published in ref. [84] and [56] .
-
Complex tissue architectures in regenerative tissues, particularly in the liver.
Examples are:
-
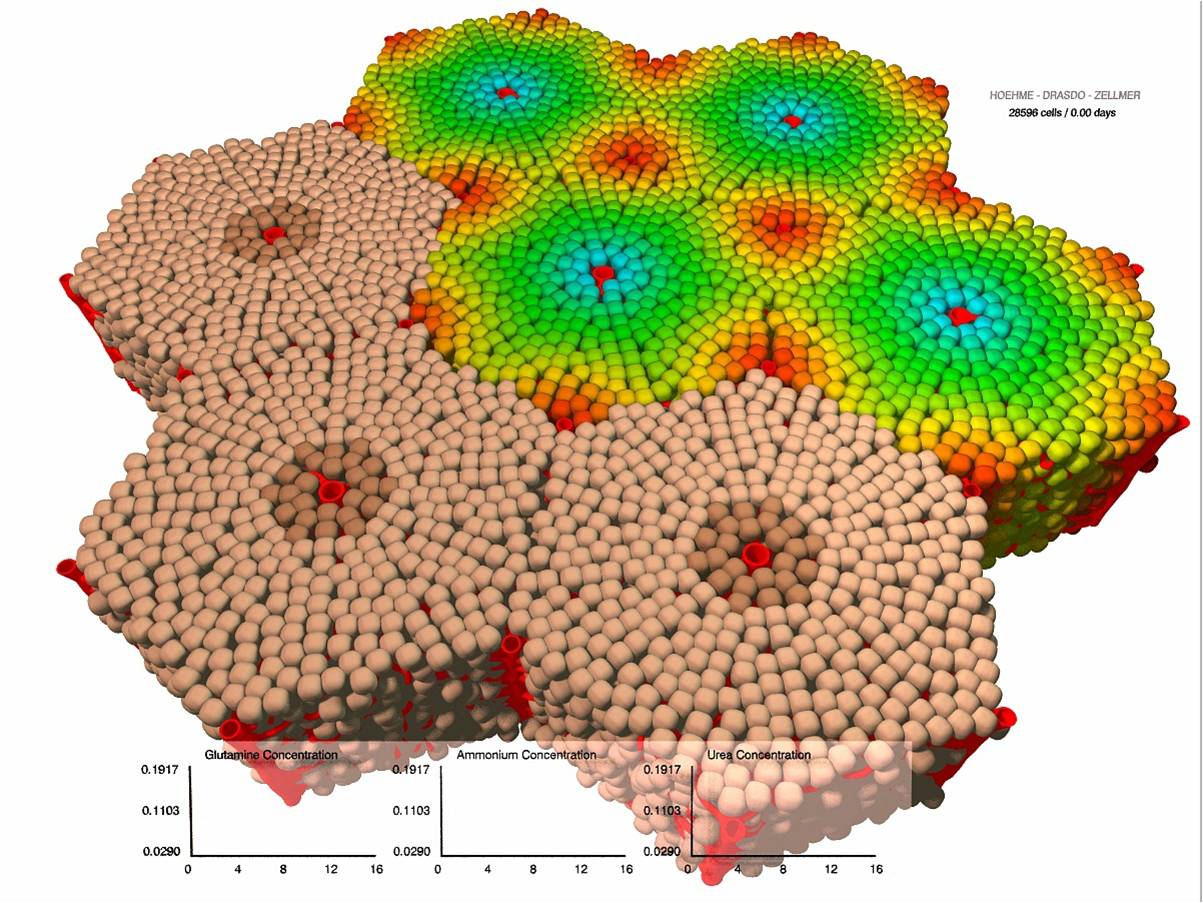
Regeneration of liver lobules after toxic damage [80] , [79] , [76] ), [40] , [41] , [42] within the German BMBF-funded network “Systems Biology of the Hepatocyte”). As extension of this project we linked the regeneration of liver architecture after toxic damage to a model of ammonia detoxification by the individual hepatocyte and the liver as a proof of concept to study the link between architecture and function. The comparison of experimental findings by our collaboration partners with our model results suggests that the detoxification during regeneration after drug-induced damage is mainly determined by the total population size of healthy hepatocytes (Fig. 2 ). Adjustments of enzymatic activities of the individual hepatocytes seem to have only minor effects.
-
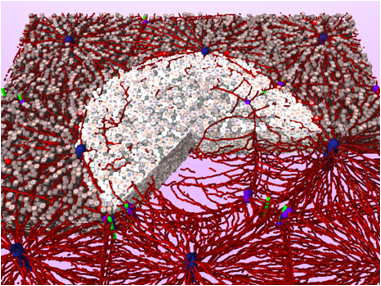
Liver regeneration after partial hepatectomy ([44] ). Based on the work on regeneration of a liver after toxic damage where we focused on a single liver lobule, we within the EU project CANCERSYS set up a model on liver regeneration after partial hepatectomy enabling us to model up to the whole liver lobe scale of a mouse, 4 cells thick. This models permits to bridge the gap between the single-cell-model scale and the whole-liver organ scale. Calibrating this model with mouse data we were able to predict the proliferation pattern in pig as a proof of principle that modelling can be used to bridge the gap between different animals. Experiments performed so far confirm the prediction. This is a fundamental issue as it is a longstanding unsolved question in how far experiments in animal models can be used to predict therapeutic responses in the Human. We also expanded the software towards whole liver lobe bright field image analysis.
Being able to use a mathematical model calibrated with data from a model animal, for example, mouse to predict tissue organisation processes in another animal, for example, human opens new possibilities to assess drug toxicity [43] .
-
The fundamental objective is to move towards modelling from the molecular up to the whole organ scale. A conceptual framework for this was presented in ref. [23] .
-
Cancer development in an environment of granular particles and cells. For the first part we studied how an embedding medium such as granular particles or cells modify the spatial and temporal growth pattern of expanding cell populations [19] . The model could explain the growth kinetics found by Helmlinger et. al. (1997) for growing tumor spheroids embedded in agarose gel. If the friction between the embedding objects and the environment is larger than the friction between the growing clone and its environment we found a fingering instability reminiscent of a Saffman-Taylor instability observed in a Hele-Shaw cell. We systematically studied which model parameters promote the instability. The motivation for this project was to analyse in how far invading tumour fronts can be explained by physical mechanisms alone as in some tumour phenotypes invasive fronts can be observed.
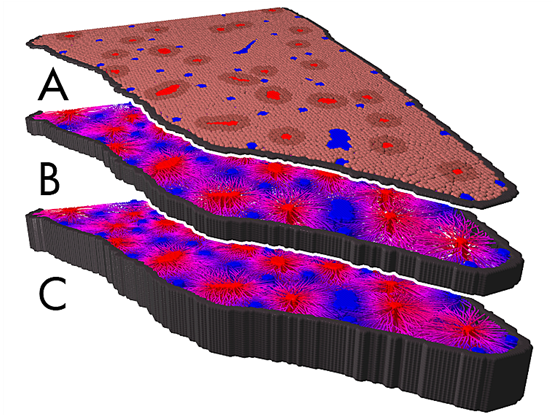
In order to extend this project towards carcinoma development mechanisms of liver cancer development in mice were studied [42] . Within systematic sensitivity analyses we were able to identify parameters explaining the experimentally found tumor phenotypes (Fig. 3 ). The critical parameters were stiffness of sinusoids, the micro-blood vessels within the liver lobules, tumor cell - sinusoidal adhesion, tumor cell polarity, and the ability of tumour cells to digest neighbouring vessels. As part of the project, in close collaboration with experimental partners, critical differences between liver in normal and transgenic mice have been studied by image analysis [62] .
-
Synthetic biology. By multi-scale simulations including intracellular pathways in our single-cell-based simulation framework we were able to mimic conditions under which tissue homeostasis and tissue location could be achieved in monolayer culture.
The applications are guided by quantitative comparisons to experimental data either from published knowledge or - in most cases - generated by experimental partners. One main focus is on the understanding of mechanisms that control the growth dynamics and growth phenotypes of multi-cellular systems and use these later to predict and optimise therapy or biotechnological growth processes.
The adjustment of the models developed to applications requires data analysis both, of molecular data such as gene expression profiles and of image data such as spatial-temporal growth pattern. For this purpose we recently considered the geometric and topological measures to quantify tumour shapes [92] , and developed an image processing chain to quantitatively analyse liver regeneration processes in liver lobules [79] , [76] , [44] , [40] , [41] . As a further step we published executables and descriptions of important elements of our code to spread our model as it turns out that agent-based cell modelling enjoys increasing interest in different communities (engineering, mathematical biology, systems biology, physics) [81] . Current directions moreover include a stronger focus on models of in-vivo systems (within the German medical systems biology consortium “LungSys” (lung cancer treatment); and within the EU-network “CancerSys” (cancerogenesis in liver)). Within LungSys we recently developed a realistic 2D and 3D spatial temporal model of blood flow in xenografts to compare to DCE MRI images visualising the tumour perfusion. Modelling cancer development requires to take into account invasion, mutations and angiogenesis, three hallmarks of cancer and of linking the molecular to the multicellular scale [71] . Moreover, we extend the topic of liver regeneration to regeneration after partial hepatectomy (within the EU-project “Passport”), and extend our modelling activities to understand early embryonic development (Trophoblast development, collaboration with INRA).
Almost each of our projects is in close collaboration with experimental partners within grant projects performing experiments to permit parameterisation and validation of our models.